INDICATION and IMPORTANT SAFETY INFORMATION for MAVENCLAD® (cladribine) tablets
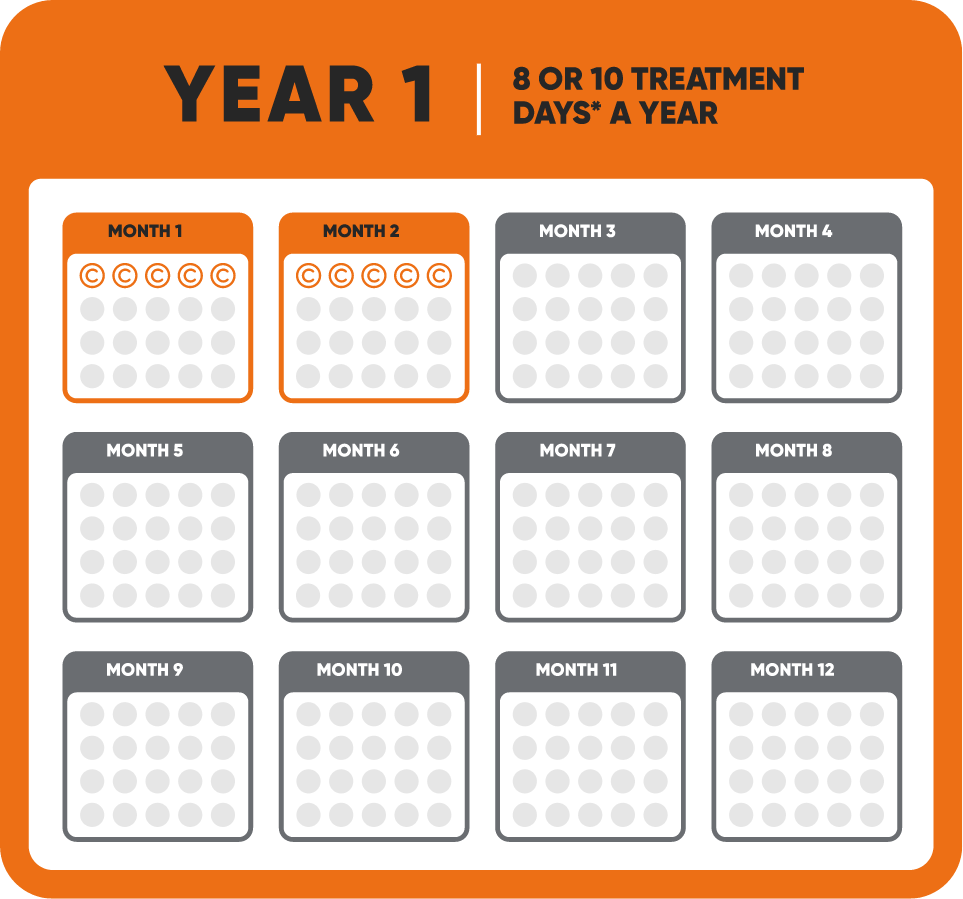
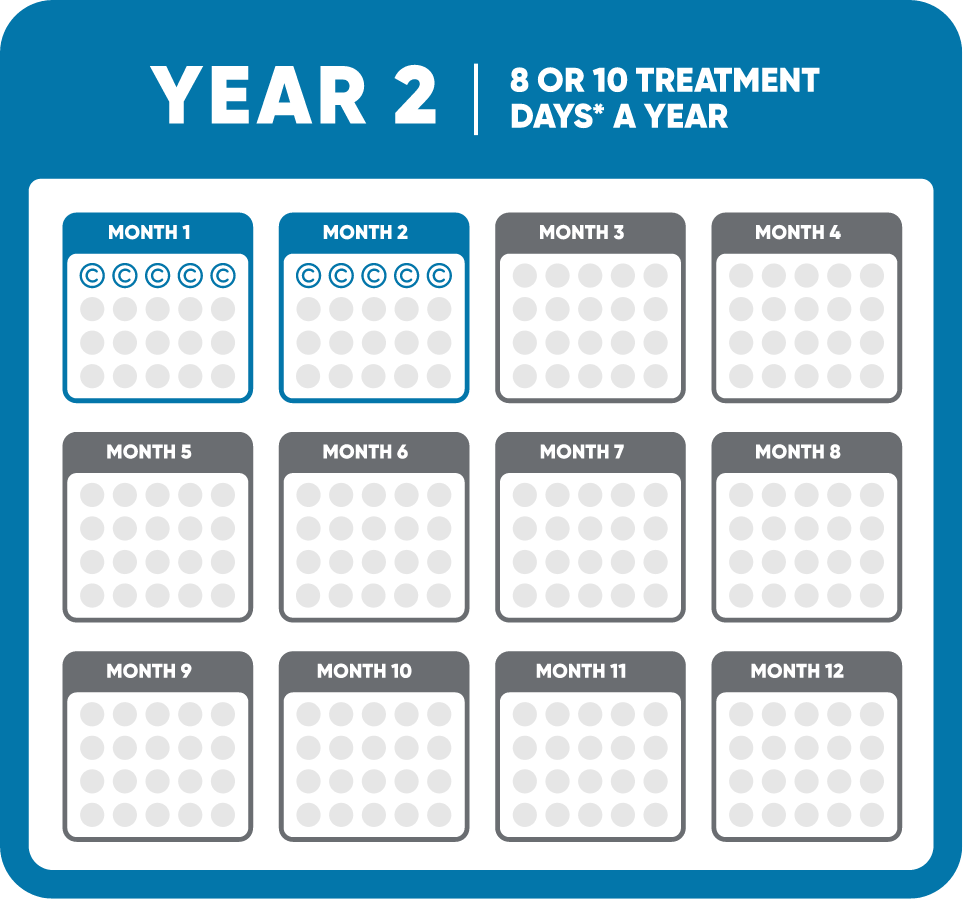
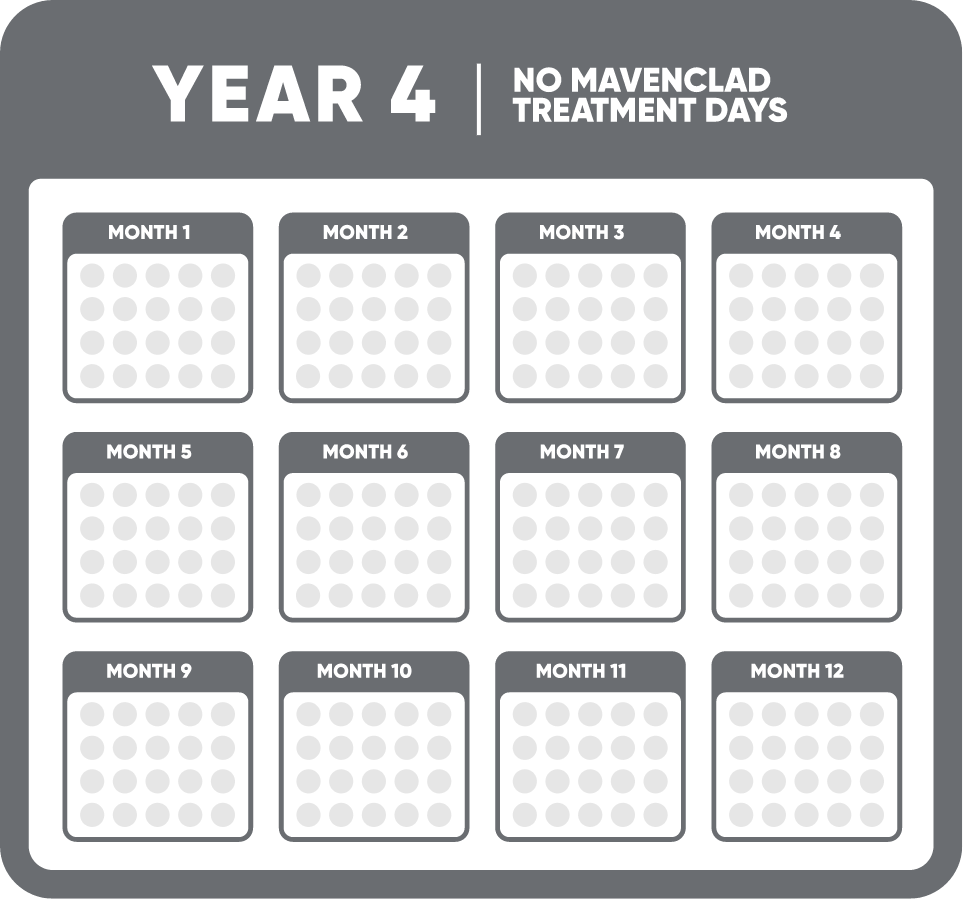
MAVENCLAD® (cladribine) is indicated for the treatment of relapsing forms of multiple sclerosis (MS), to include relapsing-remitting disease and active secondary progressive disease, in adults. Because of its safety profile, use of MAVENCLAD is generally recommended for patients who have had an inadequate response to, or are unable to tolerate, an alternate drug indicated for the treatment of MS.
Limitations of Use: MAVENCLAD is not recommended for use in patients with clinically isolated syndrome (CIS) because of its safety profile.