Four steps for taking MAVENCLAD:
Have a glass of water ready, and make sure that your hands are clean and dry before taking the tablet(s).
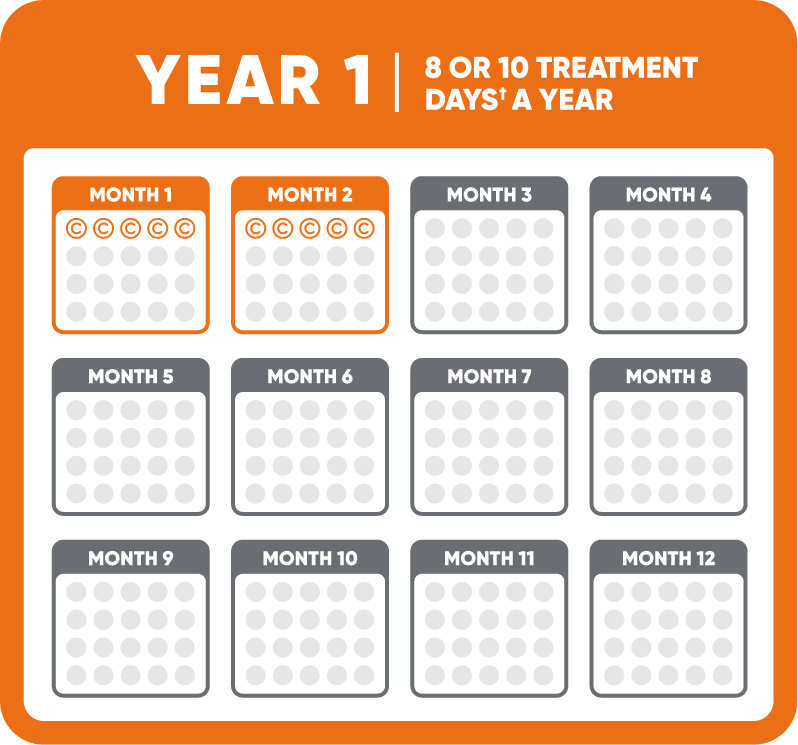
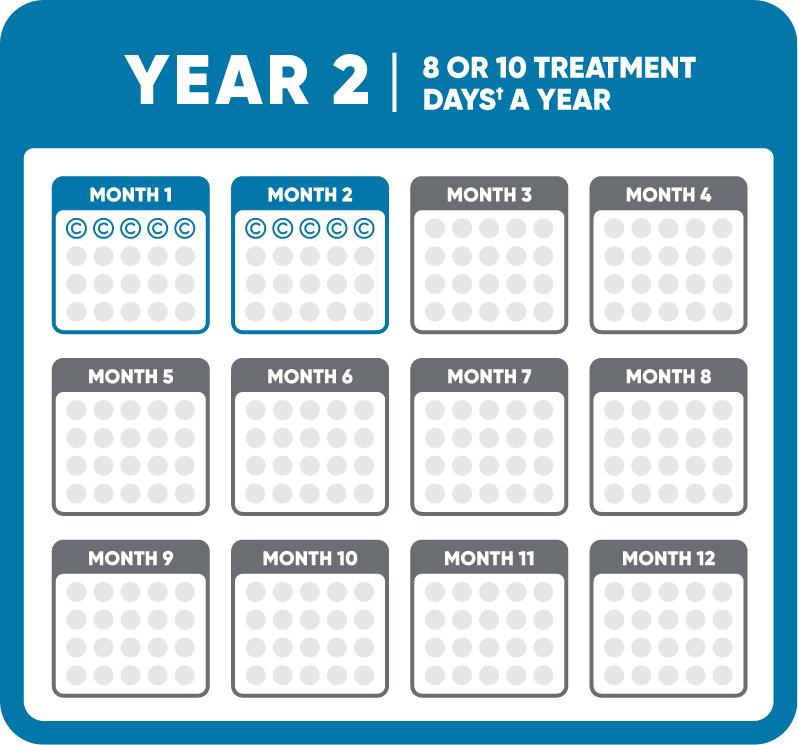
Each week of MAVENCLAD treatment, known as a cycle, consists of 1 or 2 pills a day for 4 or 5 days in a row. The number of pills you take each day depends on your weight.
There are two MAVENCLAD cycles a year, about a month apart, for 2 years. You do not need to take MAVENCLAD during Years 3 and 4.
Your health will be monitored before, during, and after treatment.
†1 or 2 pills a day depending on your weight.
Outside of this treatment schedule, you will not take any MAVENCLAD. MAVENCLAD will continue to treat your MS even when you’re not actively taking it.
Even if you feel like your MS is under control, it is important to complete the entire 2-year treatment plan as prescribed. The results seen in the clinical trial were based on completing 2 yearly treatment courses.
Your healthcare provider will continue to monitor your health during the 2 yearly treatment courses as well as between treatment courses and for at least another 2 years, during which you do not need to take MAVENCLAD.
Talk to your healthcare provider about what comes next for your treatment plan after year two.'
Your healthcare provider may delay or completely stop treatment with MAVENCLAD if you have severe side effects.
MAVENCLAD comes in a box that will include either 4- or 5-day packs. Each day pack contains 1 or 2 pills. The exact number of MAVENCLAD pills you receive is based on your weight.
It’s important to follow your healthcare provider’s instructions regarding your MAVENCLAD treatment plan.
Remember to:
Have a glass of water ready, and make sure that your hands are clean and dry before taking the tablet(s).
MAVENCLAD is a prescription medicine used to treat relapsing forms of multiple sclerosis (MS), to include relapsing-remitting disease and active secondary progressive disease, in adults. Because of its safety profile, MAVENCLAD is generally used in people who have tried another MS medicine that they could not tolerate or that has not worked well enough.
MAVENCLAD is not recommended for use in people with clinically isolated syndrome (CIS).
It is not known if MAVENCLAD is safe and effective in children under 18 years of age and is therefore not recommended.
MAVENCLAD may cause serious side effects, including:
Do not take MAVENCLAD if you:
Before you take MAVENCLAD, tell your healthcare provider about all of your medical conditions, including if you:
How should I take MAVENCLAD?
Your healthcare provider will continue to monitor your health during the 2 yearly treatment courses, and for at least another 2 years during which you do not need to take MAVENCLAD. It is not known if MAVENCLAD is safe and effective in people who restart MAVENCLAD treatment more than 2 years after completing 2 yearly treatment courses.
MAVENCLAD can cause serious side effects. If you have any of these symptoms listed below, call your healthcare provider right away:
The most common side effects of MAVENCLAD include: upper respiratory infection, headache, and low white blood cell counts.
These are not all the possible side effects of MAVENCLAD. Call your doctor for medical advice about side effects. To report SUSPECTED ADVERSE REACTIONS, contact EMD Serono at: 1-800-283-8088 ext. 5563 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see full Prescribing Information and Medication Guide, including serious side effects.