Exploring different treatment options for multiple sclerosis can feel overwhelming. That’s why understanding how MAVENCLAD is thought to work... understanding how the treatment is thought to work can help you and your doctor decide what’s right for you.
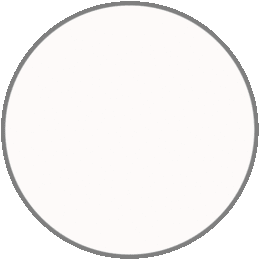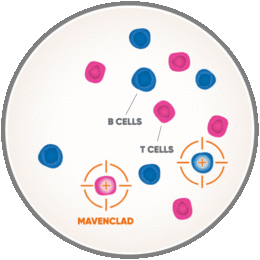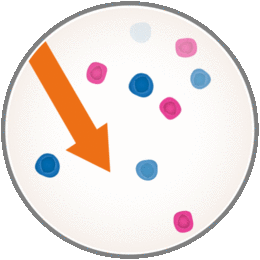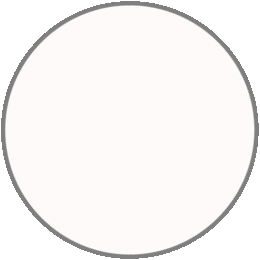
I’m Dr. Aaron Boster, the Medical Director for the Boster Center for Multiple Sclerosis. I want to tell you about MAVENCLAD, which has a dosing schedule that’s different from other RMS therapies. MAVENCLAD is an oral therapy that you take for no more than 10 days a year for 2 years. It’s the short-course dosing schedule that allows for non-continuous immunosuppression, which means MAVENCLAD only temporarily weakens your immune system. To understand how MAVENCLAD is thought to work, first let me share a few details about your immune system. Your immune system is made up of many different cells, including B and T cells, that defend your body against bacterial and viral infections.
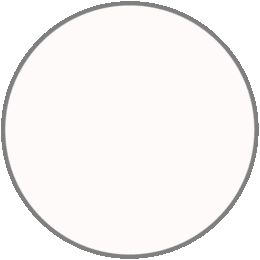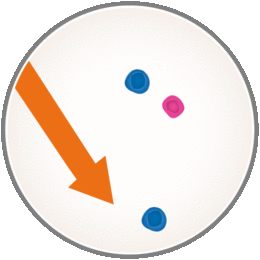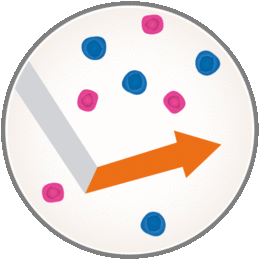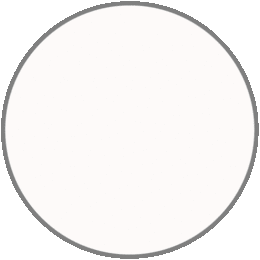
But when you have MS, B and T cells attack normal, healthy tissues of the central nervous system. This causes inflammation and damage which show up as MS-related symptoms. Now with MAVENCLAD, it’s thought to work by selectively targeting and temporarily reducing the number of B and T cells circulating in your body.
MAVENCLAD targets B cells, including those that may have an impact on MS. This means there are fewer such cells attacking the central nervous system. So when it comes to fighting infections, even when your body has a smaller number of these cells, you are still able to respond to vaccinations. Also, other immune cells, such as monocytes and neutrophils, are present to fight infections.
However, you should know that MAVENCLAD can lower white blood cell counts, which may increase the likelihood of infections during treatment. The short-course dosing of MAVENCLAD allows B and T cells to recover, or repopulate, after treatment courses. The new cells may be less likely to cause inflammation associated with MS. This means with MAVENCLAD your immune system is only temporarily weakened due to non-continuous immunosuppression.
With MAVENCLAD, your immune system may continue to fight infections and respond to vaccinations between and after each treatment course. Your doctor will continue to monitor your health during the 2 yearly treatment courses and for at least another 2 years, during which you do not need to take MAVENCLAD.
Now that we know how MAVENCLAD is thought to work with your immune system, it’s important to keep in mind that when you’ve finished your treatment for the year, MAVENCLAD will continue to treat your MS–even when you’re not taking it. Of course, everyone’s MS is different, so your experience may vary.
Hearing personal experiences from others with MS is helpful when making big decisions. So head over to MAVENCLADstories.com to hear what real MAVENCLAD patients have to say. And if you want an oral treatment that doesn’t continuously weaken your immune system, talk to your doctor about MAVENCLAD.
MAVENCLAD (cladribine) tablets
Indication and Important Safety Information
WHAT IS MAVENCLAD?
MAVENCLAD is a prescription medicine used to treat relapsing forms of multiple sclerosis (MS), to include relapsing-remitting disease and active secondary progressive disease, in adults. Because of its safety profile, MAVENCLAD is generally used in people who have tried another MS medicine that they could not tolerate or that has not worked well enough.
MAVENCLAD is not recommended for use in people with clinically isolated syndrome (CIS). It is not known if MAVENCLAD is safe and effective in children under 18 years of age and is therefore not recommended.
MAVENCLAD may cause serious side effects, including:
· Risk of cancer (malignancies). You should follow healthcare provider instructions about screening for cancer.
· MAVENCLAD may cause birth defects if used during pregnancy. Women must not be pregnant when they start treatment with MAVENCLAD or become pregnant during MAVENCLAD dosing and within 6 months after the last dose of each yearly treatment course. You should stop treatment with MAVENCLAD and contact your healthcare provider right away if you become pregnant during treatment with MAVENCLAD.
o For women who are able to become pregnant:
§ Your healthcare provider should order a pregnancy test before you begin your first and second yearly treatment course of MAVENCLAD to make sure that you are not pregnant.
o Ask your healthcare provider which contraceptive method is right for you. Women and men being treated with MAVENCLAD should use effective birth control (contraception) on the days on which they take MAVENCLAD and for at least 6 months after the last dose of each yearly treatment course.
Do not take MAVENCLAD if you:
· have cancer (malignancy).
· are pregnant, plan to become pregnant, or are a woman of childbearing age or a man able to father a child and you are not using birth control.
· are breastfeeding.
· are human immunodeficiency virus (HIV) positive.
· have active infections, including tuberculosis (TB), hepatitis B or C.
· are allergic to cladribine.
Before you take MAVENCLAD, tell your healthcare provider about all of your medical conditions, including if you:
· think you have an infection.
· have taken, take, or plan to take medicines that affect your immune system or blood cells, or other treatments for MS. Certain medicines can increase your risk of getting an infection.
· have had a recent vaccination or are scheduled to receive any vaccinations. You should not receive live or live-attenuated vaccines within the 4 to 6 weeks preceding treatment with MAVENCLAD or receive these types of vaccines during your treatment with MAVENCLAD and unless directed by your healthcare provider.
· have heart failure.
· have or have had cancer.
· have liver or kidney problems.
· are breastfeeding or plan to breastfeed. It is not known if MAVENCLAD passes into your breast milk. Do not breastfeed on the days on which you take MAVENCLAD, and for 10 days after the last dose.
How should I take MAVENCLAD?
· MAVENCLAD is given as two yearly treatment courses, consisting of 2 treatment weeks (cycles) about a month apart.
· Handle MAVENCLAD with dry hands and take immediately after opening the blister pack. Take with water and do not chew the tablet. MAVENCLAD can be taken with or without food and should be taken at least 3 hours apart from other medicines.
· Wash your hands after handling MAVENCLAD. Limit contact with your skin (especially on your face). Wash skin and surfaces with water if contact occurs.
· If you miss a dose, take it as soon as you remember on the same day. If the whole day passes before you remember, take your missed dose the next day. Do not take 2 doses at the same time. Instead, you will extend the number of days in that treatment week.
Your healthcare provider will continue to monitor your health during the 2 yearly treatment courses, and for at least another 2 years during which you do not need to take MAVENCLAD. It is not known if MAVENCLAD is safe and effective in people who restart MAVENCLAD treatment more than 2 years after completing 2 yearly treatment courses.
Your healthcare provider will continue to monitor your health during the 2 yearly treatment courses, and for at least another 2 years during which you do not need to take MAVENCLAD. It is not known if MAVENCLAD is safe and effective in people who restart MAVENCLAD treatment more than 2 years after completing 2 yearly treatment courses.
MAVENCLAD can cause serious side effects. If you have any of these symptoms listed below, call your healthcare provider right away:
· low blood cell counts have happened and can increase your risk of infections during treatment with MAVENCLAD. Blood tests are needed before you start treatment with MAVENCLAD, during your treatment with MAVENCLAD, and afterward, as needed.
· serious infections such as:
o life-threatening or fatal infections caused by bacteria, viruses, parasites or fungi.
o TB, hepatitis B or C, and shingles (herpes zoster). Fatal cases of TB and hepatitis have happened with cladribine during clinical studies. Tell your healthcare provider right away if you get any symptoms of the following infection related problems or if any of the symptoms get worse, including fever, aching painful muscles, headache, feeling of being generally unwell, loss of appetite, burning, tingling, numbness or itchiness of the skin in the affected area, skin blotches, blistered rash, or severe pain.
o progressive multifocal leukoencephalopathy (PML). PML is a rare brain infection that usually leads to death or severe disability. Although PML has not been seen in MS patients taking MAVENCLAD, it may happen in people with weakened immune systems. Tell your healthcare provider right away if you have any new or worsening neurologic signs or symptoms. These may include: weakness on 1 side of your body, loss of coordination in your arms and legs, decreased strength, problems with balance, changes in your vision, changes in your thinking or memory, confusion, or changes in your personality.
· liver problems. Symptoms of liver problems may include: nausea, vomiting, stomach pain, tiredness, loss of appetite, dark urine, or your skin or the whites of your eyes turn yellow. Your doctor will perform blood tests to check your liver during treatment.
· allergic reactions (hypersensitivities). You should stop treatment and seek immediate medical attention if any signs or symptoms of allergic reactions occur. Symptoms of an allergic reaction may include: skin rash, swelling or itching of the face, lips, tongue or throat, or trouble breathing.
· heart failure. MAVENCLAD may cause heart failure, which means your heart may not pump as well as it should. Call your healthcare provider or go to the closest emergency room for medical help right away if you have any signs or symptoms such as shortness of breath, a fast or irregular heartbeat, or unusual swelling in your body.
The most common side effects of MAVENCLAD include: upper respiratory infection, headache, and low white blood cell counts.
These are not all the possible side effects of MAVENCLAD. Call your doctor for medical advice about side effects. To report SUSPECTED ADVERSE REACTIONS, contact EMD Serono at: 1-800-283- 8088 ext. 5563 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see full Prescribing Information and Medication Guide, including serious side effects.